Discover the last paper published by Eindhoven University of Technology (TU/e) and Tecnalia within the C2Fuel project!
Carbon molecular sieve membranes for water separation in CO2 hydrogenation reactions: Effect of the carbonization temperature
Serena Poto, A. Aguirre, F. Huigh, Margot Anabell Llosa-Tanco, David Alfredo Pacheco-Tanaka, Fausto Gallucci, M. Fernanda Neira d’Angelo
Journal of Membrane Science, Volume 677, 5 July 2023, 121613 – https://doi.org/10.1016/j.memsci.2023.121613
Abstract:
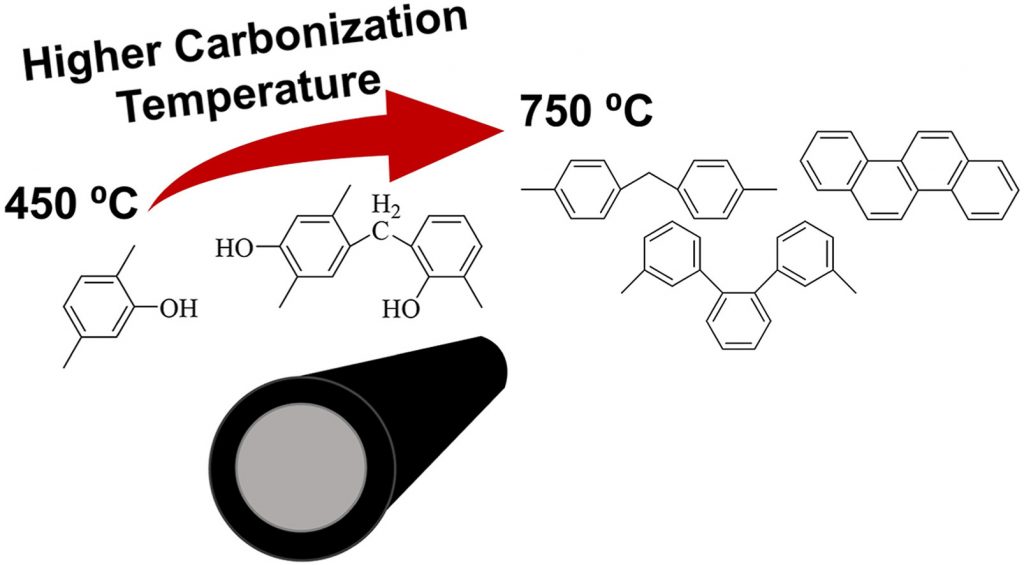
Carbon membranes are a potentially attractive candidate for the in-situ removal of water vapor in CO2 hydrogenation reactions. Their hydrophilicity and pore structure can be tuned by properly adjusting the synthesis procedure. Herein, we assess the effect of the carbonization temperature (450–750 °C) on the performance of supported CMSM in terms of vapor/gas separation, in correlation with changes in their surface functionality and porous structure. FTIR spectra showed that the nature of the functional groups changes with the evolution of the carbonization step, leading to a gradual loss in hydrophilicity (i.e., OH stretching disappears at Tcarb ≥ 600 °C). The extent of water adsorption displays an optimum at Tcarb of 500 °C, with the membrane carbonized at 650 °C being the least hydrophilic. We found that the pore size distribution strongly influences the water permeance. At all Tcarb, adsorption-diffusion (AD) is the dominant transport mechanisms. However, as soon as ultra-micropores appear (Tcarb: 600–700 °C) molecular sieving (MS) contributes to an increase in the water permeance, despites a loss in hydrophilicity. At Tcarb ≥ 750 °C, MS pores disappear, causing a drop in the water permeance. Finally, the permeance of different gases (N2, H2, CO, CO2) is mostly affected by the pore size distribution, with MS being the dominant mechanism over the AD, except for CO2. However, the extent and mechanism of gas permeation drastically change as a function of the water content in the feed, indicating that gas/vapor molecules need to compete to access the pores of the membranes.