Kinetic modelling of the methanol synthesis from CO2 and H2 over a CuO/CeO2/ZrO2 catalyst: The role of CO2 and CO hydrogenation
Serena Poto, Damian Vico van Berkel, Fausto Gallucci, M.Fernanda Neira d’Angelo
Chemical Engineering Journal, Volume 435, Part 2, 2022, 134946
https://doi.org/10.1016/j.cej.2022.134946
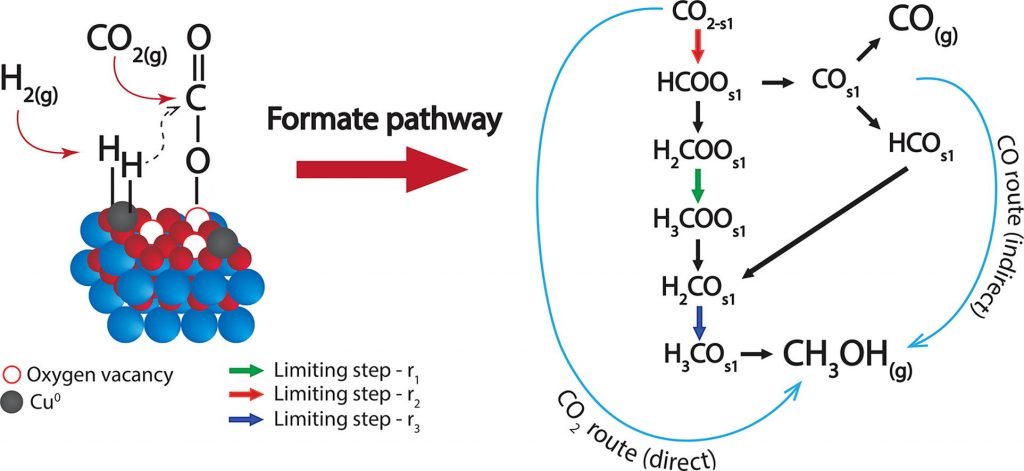
Abstract: This work addresses the kinetics of the CO2 hydrogenation to methanol over a Cu/CeO2/ZrO2 catalyst studied using single-site, dual-sites and three adsorption sites kinetic models. Physicochemical constraints and statistical indicators are used as tool for model discrimination. The best performing model is used to elucidate the reaction mechanism and the relative roles of the Cu-sites and oxygen vacancies. The results show that the dissociative adsorption of H2 occurs on the Cu0 sites, while CO2 is attracted to the oxygen vacancies created by the CeO2-ZrO2 solid solution. Then, the adsorbed H interacts preferentially with the carbon atom, favouring the so-called “formate” route. The CO formed via the r-WGS reaction could either desorb to the gas phase or react via hydrogenation to methanol. Analysis of the relative contributions of the CO2 and CO hydrogenation (i.e. direct and indirect pathways, respectively) to the methanol synthesis reveals that the latter is in fact preferential at high temperatures (i.e. about 100% of methanol is produced from CO at 260 ⁰C and 30 bar), and it shows an optimum vs the H2:CO2 ratio (c.a. 7 at 200 ⁰C and 30 bar), which corresponds to the saturation of the Cu0 sites with H2. Thus, this work provides an essential tool (i.e., kinetic model) for the design of reactors and processes based on novel catalysts, and importantly, it offers a deeper understanding of the reaction mechanism as basis for further catalyst development.
Keywords: CO2 conversion; Methanol synthesis; Kinetic modelling; Oxygen vacancies; Cerium-zirconium oxides